Lead drugs, scientists, publications & trials
LSALT peptide, is the company’s lead drug candidate targeting acute kidney injury and other organ inflammation injuries in the kidneys, lungs and liver.
LSALT peptide trials and development
The company is the sponsor of a Phase II international multi-center, randomized, double-blind, placebo-controlled study of LSALT peptide targeting cardiac surgery-associated acute kidney injury (CS-AKI). Drug approval for treating this type of inflammation related kidney injury may enable off-label use for other indications such as lung or liver inflammation injury, other AKI indications and septic shock.

Click to WATCH LSALT peptide At work
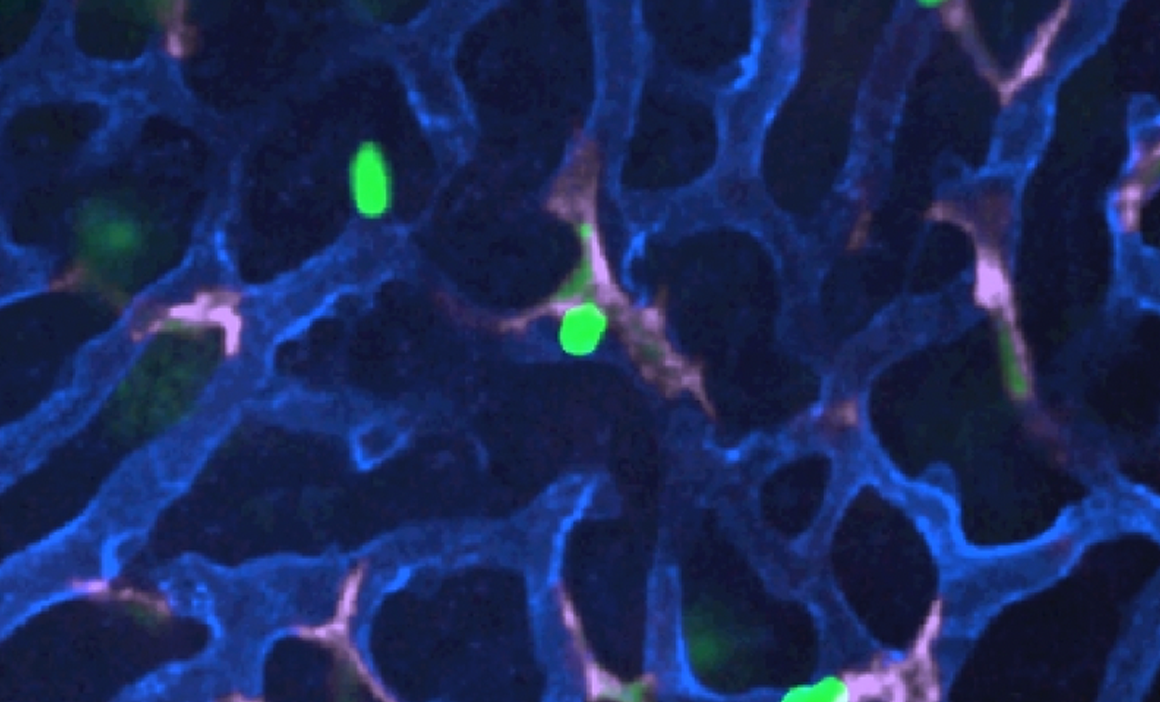
Video data behind Arch lead scientists publication in Cell shows the real-time effects of LSALT peptide when used to prevent inflammation in pre-clinical models of AKI, similar to the inflammation found in CS-AKI – click to watch the videos and learn more about LSALT peptide.
The CS-AKI trial began dosing patients in March 2024. Starting with five hospital sites in Turkey, one active site in Calgary and two additional sites announced in Toronto (pending activation). The complete study is planned to include up to 240 patients in Turkey, Canada and the United States.
- November 2024, announcement of dosing of the first patient dosed at the University of Calgary, Cumming School of Medicine, the first Canadian site to join and activate in the trial.
- April 2024, announcement of St Michael’s Hospital and Toronto General Hospital (UHN) joining the trial.
- March 2024, dosing of first patient in Turkey marking the commencement of the Phase II CS-AKI trial.
- March 2024, publication of previous Phase II trial results targeting DPEP1 mediated lung inflammation provides further scientific rationale to advance LSALT peptide to prevent leukocyte recruitment and organ inflammation injury for other indications.
Find out more about our science platforms and about LSALT peptide clinical trials and development targeting the treatment and prevention of common injuries and diseases related to organ inflammation.
Cilastatin is Arch’s second drug asset targeting acute kidney injury (AKI)
a DPEP1 inhibitor suited to preventing AKI caused by toxins
In 2024, the company announced that it is repurposing cilastatin and working with research and clinical collaborators to develop the drug as a new treatment to prevent toxin-related AKI.
Cilastatin was originally developed in the early 1980s by Merck Sharp & Dohme Research Laboratories to limit DPEP1’s role in the breakdown of imipenem, a β-lactam antibiotic used for the treatment of systemic infections. Now off patent, Arch is producing cilastatin to be approved for the first time as a standalone drug product.
- Arch has method-of-use patents for cilastatin as a treatment to prevent kidney injury – currently issued or pending
- Cilastatin is particularly well suited to prevent AKI caused by drug toxins due to its unique off-target effects that block toxin uptake into the kidney tissue
- In August 2024, Arch announced that the company is participating in the PONTiAK phase II trial targeting acute kidney injury caused by drug toxins
AKI reflects a broad spectrum of clinical presentations ranging from mild injury to severe injury that may result in permanent and complete loss of renal function. Clinically, the causes of AKI include sepsis, ischemia-reperfusion injury, and various endogenous as well as exogenous (drug) toxins. There is no specific therapeutic treatment available in the market today that prevents AKI. In the worst cases, the kidneys fail, requiring dialysis or kidney transplantation for patient survival.
Patents for imipenem and cilastatin have expired and the combination drug is currently in a generic phase. There is no commercial history of cilastatin as a stand-alone drug product.
An experienced group of scientists working together to develop the Arch science platforms.
Science and Research Teams
- Current work on LSALT peptide and Cilastatin is directed by Arch’s larger Treatments for Inflammation Team, led by Dr. Daniel Muruve (Arch Biopartners CSO), Dr. Justin Macdonald Ph. D., and Dr. Arthur Lau Ph. D (Arch Biopartners Project Director), who are primarily based at the University of Calgary. David Luke, BScPhm, PharmD, is a Strategic Advisor and Senior Clinical Lead working to support the Board of Directors and the company’s ongoing clinical trials with LSALT peptide.
- The DPEP1 Inflammation Pathway and LSALT Peptide (Metablok) were discovered by the Metablok team – Dr. Stephen Robbins Ph. D., Dr. Donna Senger Ph. D., Jennifer Rahn B Sc., M Sc. and their colleague Dr. Paul Kubes at the University of Calgary.
The Arch Biopartners Science Teams have all participated and authored key papers and journal publications as a core part of their ongoing scientific research, discoveries and development.
- Treatments for Inflammation publications surrounding the development of LSALT Peptide as a treatment for inflammation with broad application to prevent organ and tissue injury
- BMJ Open, March 2024 – Multicentre, randomised, double-blind, placebo-controlled, proof of concept study of LSALT peptide as prevention of acute respiratory distress syndrome and acute kidney injury in patients infected with SARS-CoV-2 (COVID-19)
- Science Advances, February 2022 – Dipeptidase-1 governs renal inflammation during ischemia reperfusion injury
- Cell, August 2019 – Dipeptidase-1 is an Adhesion Receptor for Neutrophil Recruitment in Lungs and Liver